Understanding Bemotrizinol and Its Role in Sunscreens
Bemotrizinol là gì?
Bemotrizinol, also known by its INCI name Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine and commonly marketed as Tinosorb S, is a broad organic UV filter. Folks recognize it for its skill at taking in both UVA and UVB light. It provides complete protection from the sun. BFP-SP S stands out as a very good, oil-based organic UV filter for UVA and UVB rays. Since it ranks among the top oil-soluble choices available, it has become an important element in clever sunscreen blends in Europe, Asia, and Australia.
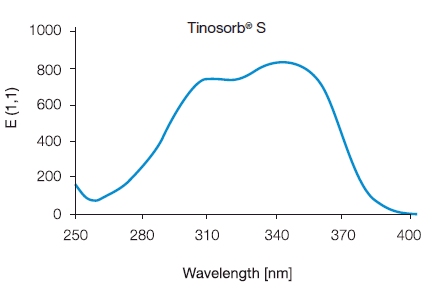
The way it keeps steady under light is a major advantage. Filters from the past fade quickly in sunlight. However, bemotrizinol stays firm. And it functions all through the time spent outdoors. This quality aids greatly in its overall effectiveness and security. On top of that, bemotrizinol pairs well with various other organic and mineral filters. Therefore, producers can create more even and robust sunscreen products.
How Bemotrizinol Works as a UV Filter
Bemotrizinol absorbs powerful ultraviolet beams. It turns them into gentle warmth. And this action blocks the harmful impacts on the skin. Sunscreens made from minerals reflect or scatter the light. Yet, the structure of bemotrizinol allows it to capture both brief and extended UV lengths quite effectively.
It acts as an aid to improve outcomes. It displays solid steadiness in light. Moreover, it ranks as a leading helper for UV filters that weaken under light. When combined with unstable components such as Avobenzone, bemotrizinol improves the complete steadiness and duration of the sunscreen. Such a partnership helps producers achieve greater SPF values. They need smaller amounts of the core elements. In the end, these sunscreens feel less heavy and simpler to apply.
Regulatory Landscape of Sunscreen Ingredients in the U.S.
The FDA’s Role in Approving UV Filters
In the United States, the Food and Drug Administration (FDA) treats sunscreens as over-the-counter (OTC) drugs. Thus, every main component, including UV filters, faces strict tests for safety and performance before gaining permission. These tests include studies on toxins, the amount absorbed into the human body, and reviews of extended exposure.
At present, just 16 UV filters hold FDA approval for OTC sunscreens. This set has remained nearly fixed for a long while. For fresh items like bemotrizinol, makers have to file an over-the-counter (OTC) monograph order request under the Tier 1 pathway. And this route suits active parts that show a proven safety and effectiveness history beyond the United States.
Even so, plenty of people claim the approval steps are overly difficult and drag on. As of 2025, bemotrizinol has not wrapped up this examination. For that reason, it lingers before entering sunscreens available in the U.S.

Comparison Between U.S. and International Regulations
The U.S. stands apart from regions such as the European Union, Australia, and certain Asian areas. Over there, they follow a more up-to-date method to greenlight sunscreen components. They permit a wider array of ., like bemotrizinol. And this decision stems from research proof supplied by producers, plus years of actual use in the field.
This item matches tough UV shield demands across the globe. In such locations, bemotrizinol turns up in sunscreens boasting high SPF. The reason lies in its full-range action and firm light resistance.
However, in the U.S., sluggish rule-making delays these modern, superior sunscreen elements. That gap has stirred up steady interest from shoppers and skin experts. They urge the FDA to refresh its guidelines. And they seek better harmony with worldwide benchmarks.
Bemotrizinol FDA Approval Status as of 2025
Current Approval Status in the United States
As of 2025, bemotrizinol was not yet FDA-approved for use in OTC sunscreen products sold within the United States. It enjoys broad application worldwide. Plus, it boasts a firm safety background. Still, it sits in ongoing rule checks. These form part of wider pushes to widen the roster of permitted UV filters.
Factors Influencing the Approval Timeline
Some elements drag out the approval steps:
Long-Term Safety Evaluations
The FDA calls for broad human safety details. This could cover research on body-wide uptake and thoughts on prolonged contact.
Need for Clinical Trials
Extra tests in living subjects often prove necessary to fulfill U.S. rule levels for OTC drug parts.
Policy Reforms
Acts like the Sunscreen Innovation Act aimed to shorten review periods. That said, shifts in rules have moved ahead gradually.
Health advocates for the public keep asking for quicker looks. They hope to draw U.S. sunscreen rules closer to practices abroad. Businesses like Shanghai BFP New Material Co., Ltd. center on providing cutting-edge UV filter parts for the worldwide sun protection and beauty sectors. They stand ready to handle growing needs if permission arrives.
Consumer Access to Sunscreens with Bemotrizinol
Availability Through Imported or Non-U.S.-Marketed Products
Even though bemotrizinol misses FDA approval, buyers can reach sunscreens that hold this part via shops from other countries or web sellers. Such goods usually blend smart sets of organic UV filters. These features are Ethylhexyl Triazone and Diethylamino Hydroxybenzoyl Hexyl Benzoate (DHHB). And Shanghai BFP delivers both under the product codes BFP-SP EHT and BFP-SP DHHB.
Designs like these often appear in waterproof sunscreens with high SPF. Plenty of sunscreens from overseas offer firmer UVA defense than those sold in the U.S. This stems from access to these recent UV filters.
Considerations When Using Non-FDA-Approved Sunscreens in the U.S.
Bringing in these goods for own use is usually allowed. But they might not follow U.S. marking or mixing rules. To cut down on dangers, shoppers ought to:
- Make sure every part gets listed plainly
- Buy from known brands and sellers
- Skip fake or wrongly marked items
Sticking to these tips can aid in safer handling of sunscreens with bemotrizinol. And that holds true even lacking local okay.
The Future Outlook for Organic UV Filters Like Bemotrizinol in the U.S. Market
Industry Push Toward Modernizing Sunscreen Regulations
Numerous parties, from skin specialists to producers, go on pressing for tweaks to FDA sunscreen approval methods. A method rooted more in facts could weigh worldwide info and long-term field experiences. In turn, that might speed up entry to fresh UV filter tools.
Outfits like Shanghai BFP New Material Co., Ltd. gear up to back fresh steps. They furnish strong organic UV filters to the world scene. Plus, they grow buying choices for forward sun protection mixes.
Potential Benefits of Expanded Filter Options for Consumers
Should bemotrizinol and like organic UV filters earn FDA approval, shoppers might gain in various ways:
| Lợi ích | Description |
| Enhanced Protection | Broad-spectrum coverage with long-lasting photostability |
| Better Skin Feel | Lightweight formulations with minimal white cast |
| Advanced Formulas | Synergistic blends that increase SPF without irritation |
| Reduced Application Frequency | Longer-lasting efficacy reduces the need for frequent reapplication |
Such upgrades would boost the general level of sun care offerings in the U.S. They would pull them in line with norms from abroad.
Tại sao UV hấp thụ quan trọng trong chăm sóc da?
Is bemotrizinol safe?
Bemotrizinol has been used safely for many years in European and Asian markets, with extensive data supporting its non-irritating and non-sensitizing profile.
What is bemotrizinol’s FDA approval status 2025?
In 2025, bemotrizinol was still not FDA-approved for use in OTC sunscreens but remains under active regulatory review.
Why are organic UV filters like bemotrizinol preferred?
They provide superior broad-spectrum protection, excellent photostability, and better cosmetic appeal compared to many older UV filters.
What are some organic UV filters examples approved globally?
Examples include Bemotrizinol (Tinosorb S), Ethylhexyl Triazone (Uvinul T150), and Diethylamino Hydroxybenzoyl Hexyl Benzoate (Uvinul A Plus).